Desalination: Ecological Doubts and Future Prospects
Filippo Verre - November 12, 2021


Desalination, or the removal of the salt fraction from water containing salt, is a scientific process that has aroused the curiosity of human beings for a very long time. It is not known exactly when the first scholars actually began to dedicate themselves to it; the fact is that one of the first to study the subject in question was Leonardo da Vinci, who since the Renaissance realized that a certain quantity of distilled water could be produced cheaply using a still and a stove. Other documented testimonies report that during a siege in Tunisia in 1560, seven hundred Spanish soldiers resisted for months against a large Ottoman army thanks to an intuition of the captain. The latter built a still capable of producing 40 barrels of fresh water per day by desalination large quantities of sea water. During the following centuries, interest in desalination became increasingly strong, but it was only from the 1960s that desalination plants began to be produced on an industrial level. The first large desalination plant was inaugurated in 1961 in Freeport, Texas. The US government was trying to mitigate the water crisis that the southern state had been going through for over a decade with a large-scale desalination process.
Nowadays, this scientific method is the basis for the production of large quantities of fresh water. In the last twenty years alone, it is estimated that in the world the water generated through desalination processes has increased by 8 times, reaching more than 100 million liters per day (1). On paper, desalination appears to be an excellent way to solve the numerous water scarcity crises that grip various countries in the global context. The premise of this is that approximately 97% of the water present on our planet comes from the seas and oceans; for 2%, even if it is drinkable or in any case suitable for domestic use, it is "trapped" in glaciers and icebergs. Only 1% of fresh water resides in lakes and rivers. Therefore, at least theoretically, the use of large-scale desalination plants could contribute to permanently solving many problems, since the salt water present in the seas is an almost inexhaustible element. In addition, according to the Stockholm International Water Institute (SIWI), by 2030 approximately 47% of the world's population could experience water supply problems. The influential Swedish think tank has been warning world public opinion, politics and international organizations for years about the serious situation that looms on the horizon in terms of water.
For some years, desalination has been considered an effective method to mitigate a situation that threatens to trigger serious tensions in the near future. Currently, the largest share of the demand for desalinated water is represented by public consumption related to the supply of drinking water to the population (61%), just under a third is absorbed by industry (29%) and the remainder is used for irrigation (2%), consumed by power plants (5%) or used for other purposes (3%). Basically, desalination plants are now present in many countries, especially in the Middle East, which use this scientific process to introduce large quantities of sea water purified from its saline component into their economy and society. However, the massive use of desalination plants entails serious side effects both on the environmental front and from a production point of view.
The “dark sides” of desalination: high production costs and little environmental sustainability
Until the early 2000s, desalination represented a valid alternative for many nations that experienced problems related to water supply. However, the widespread use of desalination plants that has characterized the last few years has posed serious enigmas. Currently, the main problems that this method of producing fresh water generates are of two types, which are closely interconnected: high production costs and little environmental sustainability.
From an energy point of view, generating drinking water on an industrial scale through desalination processes is extremely expensive. In all fairness, it should be noted that recently the technologies adopted to bring this complicated scientific process to life have made great strides, significantly reducing costs that only a few years ago were much higher. To get a clear idea of this, keep in mind that in the past, to produce a liter of desalinated water, an average of about 4 kWh were required. Currently, technological and engineering evolution has made increasingly advanced materials and increasingly efficient construction schemes available, reducing the energy requirements of the systems.
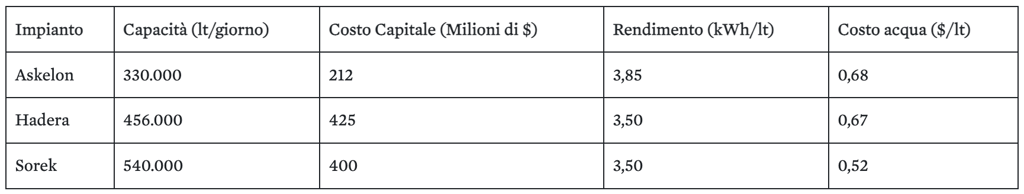
Tab. 1: Dettagli sul rendimento e sul consumo energetico dei principali dissalatori israeliani


Fig. 1: Impianto di dissalazione di Askelon


Fig. 2: Impianto di dissalazione di Hadera


Fig. 3: Impianto di dissalazione di Sorek
What is happening in Israel is a very widespread trend. In fact, many other Middle Eastern nations make extensive use of desalination plants, which are powered mainly by fossil fuels. The main reason is that these countries are rich in oil, an element that is still used massively in many production processes, including desalination. The copious use of fossil fuels to power desalination plants causes the release into the atmosphere of tens of tons of CO2 and other pollutants every year. According to a study commissioned by the United Nations, in 2017, approximately 150 million tons of CO2 were released by all the world's desalination plants. On the one hand, therefore, a lot of water usable for domestic purposes has been produced, on the other hand, the atmosphere has been significantly polluted (2).


Fig. 4: Il dissalatore Jebel Ali, principale impianto di Dubai
So, in addition to the high energy costs, it is worth noting that large-scale desalination also causes enormous environmental problems. Unfortunately, air pollution is only one of the polluting effects generated by this scientific process. In concrete terms, for every hundred liters of fresh water produced, there is a residue of 1.5 liters of brine, with variable concentrations and depending on the salinity of the starting water. Brine, together with other substances used in the desalination process, is nothing more than a real industrial residue, a waste element that, as such, must be disposed of. As previously reported, currently around one hundred million liters of desalinated water are produced per day throughout the world. This means that desalination plants, in addition to generating a lot of desalinated water, also produce around 150 million cubic meters of hyper-saline brine per day (3). Very often, waste from desalination plant activities is dumped into the sea, since disposal costs would be too high. This also causes serious environmental damage on the coastal front and in marine environments. In fact, it is not uncommon to come across industrial waste a short distance from beaches or coasts near desalination plants. Magnesium, chalk, sodium chloride, calcium chloride and potassium chloride are just some of the materials that are often found in waste. Furthermore, the hyper-saline brine that is released into the sea causes the death of many species of fish; the strong concentration of salt in small areas upsets the aquatic environment and alters the living conditions of many species. According to some biologists, even some types of marine algae (above all the Posidonia plant), essential to ensure a correct natural balance, would suffer particularly from this situation (4).
The main desalination techniques and future prospects
At the moment, reverse osmosis is the most widespread technology in large-scale plants and is the one on which the greatest short-term expectations are concentrated. According to Enrico Mariutti's analysis, approximately 50% of the plants in operation globally and 90% of those under construction or in the design phase exploit reverse osmosis processes (5). This technology uses semi-permeable membranes. These are membranes that allow the passage of only certain molecules. The flow depends on various conditions, including pressure, salt concentration, temperature; the intrinsic characteristics of the membrane also play a determining role, since they are essential for separating fresh water from solid residues and impurities that make it unsuitable for civilian consumption. Reverse osmosis requires a lot of energy to function correctly. With this technique, sea water is pushed through desalination membranes at a pressure that can reach up to 70-80 atmospheres. To do this, the use of energy must be considerable. Often, to reduce production costs, companies and states that use desalination plants use fossil fuels, which are highly efficient and available without excessive costs but, as we know, very polluting. Therefore, in the future the use of this desalination technique runs the risk of becoming unsustainable from an environmental point of view.
Other desalination techniques have recently been identified that could have a less invasive impact. One of these is the so-called membrane distillation, according to which sea water is not compressed, but heated to a few dozen degrees in order to form steam. The latter, passing through a special membrane, condenses into fresh water. This system "squeezes" even more fresh water from the sea water, until only crystallized salts are left, which therefore, instead of being discharged into the sea, are themselves a saleable product. The desalinated water produced with this technique is much purer than that produced by reverse osmosis and does not require further treatments, further reducing energy consumption. Furthermore, membrane distillation does not use electricity but low-temperature heat, around 70 °C; this temperature can be easily reached without a massive use of fossil fuels, and can even be the product of photovoltaic or geothermal systems. One of the new frontiers of desalination, in fact, is to increasingly use renewable energy sources, in order to have a low impact on the environment and contain production costs. In this regard, carbon nanotubes, graphene, solar electrolysis or other artificial solutions are being tested that essentially “suck” fresh water from sea water through the use of energy from photovoltaic panels or geothermal power plants.
_______________________________________________________________________________________________________________________________________________________________
1. Per ulteriori dettagli si rimanda a Enrico Mariutti, Dissalazione: problemi di ecosostenibilità e prospettive di breve periodo, aprile 2018 https://www.qualenergia.it/articoli/20180430-dissalazione-problemi-di-ecosostenibilita-e- prospettive-breve-periodo/
2. Ulteriori dettagli sono reperibili presso https://www.lifegate.it/vantaggi-svantaggi-dissalazione-soluzione-contro-siccita.
3. Per avere un’idea di quanto materiale di scarto venga prodotto annualmente si tenga presente che, in un anno, la salamoia venutasi a creare sarebbe sufficiente a coprire metà della superficie italiana sotto 30 centimetri di melma caustica. Cfr. Luigi Bignami, in «Focus», ottobre 2019, https://www.focus.it/ambiente/ecologia/dissalatori-di-acqua-di-mare-e-salamoie.
4. Dario Zerbi, Vantaggi e svantaggi della dissalazione, una soluzione contro la siccità, in Lifegate, novebre 2018, https://www.lifegate.it/vantaggi-svantaggi-dissalazione-soluzione-contro-siccita.
5. Dario Zerbi, Vantaggi e svantaggi della dissalazione, una soluzione contro la siccità, in Lifegate, novebre 2018, https://www.lifegate.it/vantaggi-svantaggi-dissalazione-soluzione-contro-siccita.
Abaqua
Via Cassia, 615
00189 Roma (RM)
© 2024. All rights reserved.
Codice Fiscale: 96584590580


